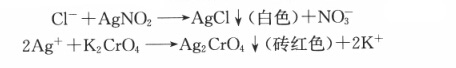I. Determination of Total Nickel in Nickel Sulfate and Nickel Chloride
1.Method summary:
In alkaline solution, nickel and EDTA form a stable complex with ammonium uramonate as indicator. The reaction is as follows:
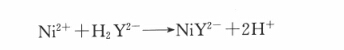
(1) Standard 0.05mol EDTA solution;
(2) Buffer solution (pH = 10): Dissolve 54g of ammonium chloride in water, add 350mL of ammonia (d = 0.89) and dilute to 1L with water;
(3) Ammonium thiazolate indication Agent.
3.Analytical method;
Take 10mL of analytical plating solution in a 100-volume flask, dilute to the mark with water, and shake. Pipette 10 mL of this dilution into a 250 mL Erlenmeyer flask, add 80 mL of water, and 10 mL of buffer solution. Add a small amount of ammonium urea indicator and titrate with standard 0.05 mol EDTA until the color changes from orange to purple.
II. Determination of chloride ion content in nickel chloride
1.Method summary;
Chloride ion and silver nitrate quantitatively generate a white silver chloride precipitate with potassium chromate as the indicator. The reaction formula is as follows:
2.Analytical method;
Pipette 10 mL of plating solution into a 100 mL volumetric flask, dilute to the mark with water, and shake. Pipette 10 mL of this dilution into a 250 mL Erlenmeyer flask (if the total amount of Ni2 is diluted, the diluent can be drawn directly). Add 50 mL of water and 2 to 5 drops of a 1% solution of potassium chromate, and titrate with 0.1 mol of standard silver nitrate until the last drop of silver nitrate produces a slightly pale red precipitate.
Third, the determination of boric acid
1.Method summary;
Boric acid is a weak acid and cannot be titrated directly with alkali. However, glycerol, mannitol, and invert sugars, which contain polyhydric organics, can form strong complexes with boric acid, which can be titrated with alkali, using phenolphthalein as an indicator.
2. Reagents;
(1) Glycerin mixture: 60g of sodium citrate is dissolved in a small amount of water, add 600 mL of glycerol, add 2 g of phenolphthalein (dissolved in a small amount of warm ethanol), and dilute to 1L with water.
(2) Standard 0.1 mol sodium hydroxide solution.
3.Analytical methods;
Pipette 10 mL of the diluted solution into a 250 mL Erlenmeyer flask, add 10 mL of water, add 25 mL of glycerol mixture, and titrate with 0.1 mol of standard sodium hydroxide until the solution turns from light green to grayish blue.
Note: The end numbers are light green-gray blue-purple. For example, the end of grayish blue is not easy to control and can be dripped to purple red minus the excess ml (about 0.2 mL). .